Standards & Philosophies
Whether in the pharmaceutical industry, the reprocessing of medical devices, the establishment of a pharmaceutical wholesaler or in the semiconductor industry, regulatory compliance always refers to compliance with all relevant laws, regulations, standards and guidelines that are applicable to production and sales and the use of these technologies apply.
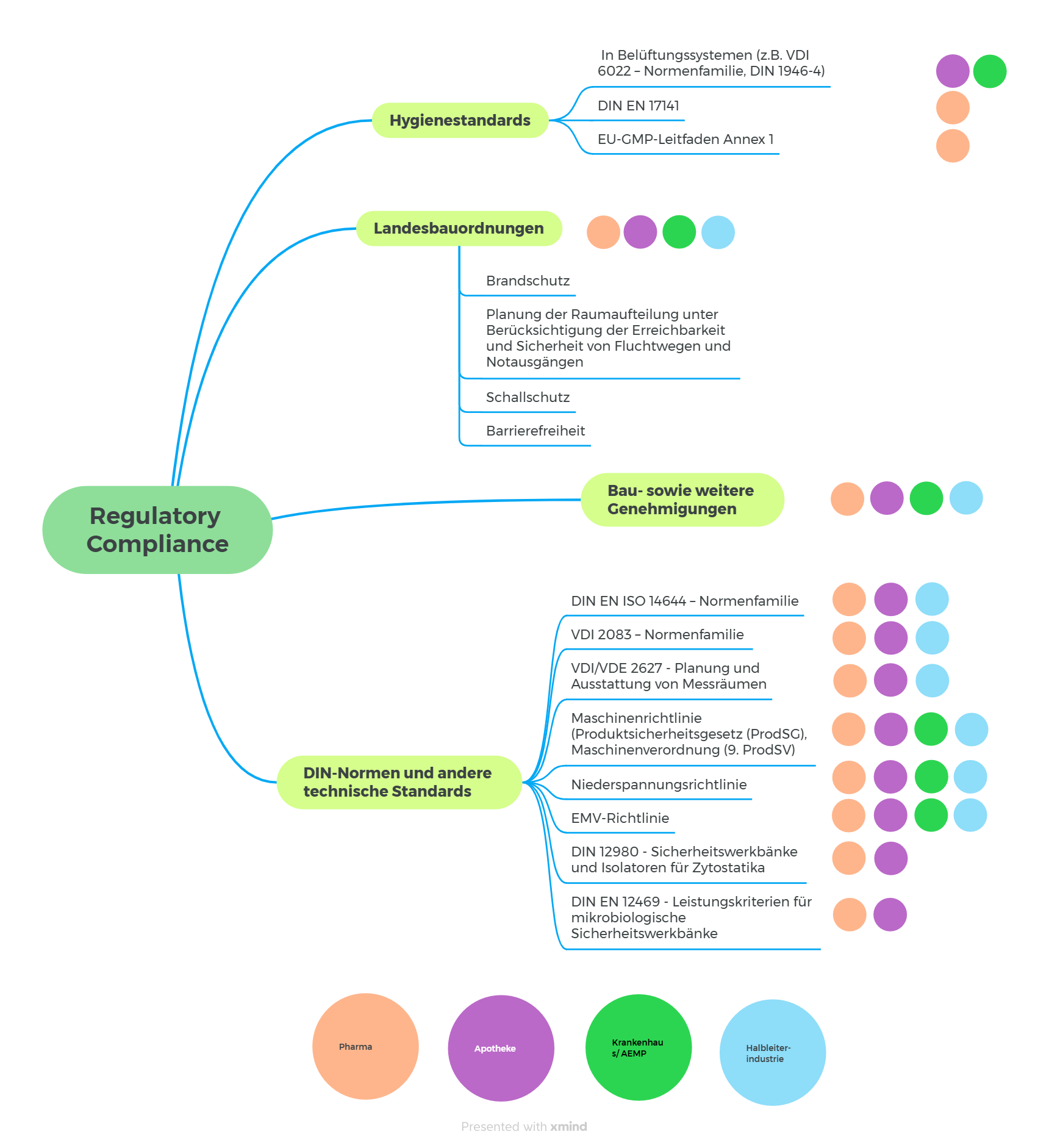
For us at P³, this regulatory compliance is crucial to ensure that clean rooms in particular are designed to be safe, functional, sustainable and legally compliant. To achieve this, a large number of regulations and standards must be adhered to, from the first project phases and throughout all planning and execution phases, which affect the entire construction process, from planning to material selection and execution. Only by taking these requirements into account can quality and safety in the project be guaranteed and legal risks minimized at the same time.
Careful documentation of all processes and tests during planning and execution ensures the traceability and reproducibility of the results.
Regular building inspections to check compliance with regulations are a given.
In order to comply with documentation obligations, we at P³ believe strongly in the use of format templates and checklists.
In our customer projects, we were able to efficiently and effectively address the challenges that we continually face in projects using a variety of standardized templates.
Call us! We would also be happy to provide you with a solution.
Linklist: Guidelines & Co.
Pharmaceuticals
Gesetz über den Verkehr mit Arzneimitteln – AMG
Arzneimittel- und Wirkstoffherstellungsverordnung – AMWHV
EU-GMP-Leitfaden – deutsche Übersetzung des BMG (zur AMWHV)
EudraLex – Volume 4 – Good Manufacturing Practice (GMP) guidelines
FDA Current Good Manufacturing Practice (CGMP) Regulations
WHO – Health products policy and standards
ZLG: Rechtliches und grundlegende Dokumente
Medical devices
Medizinprodukte-Betreiberverordnung – MPBetreibV
RKI – Aufbereitung von Medizinprodukten (2012)
DGSV – Empfehlungen des Fachausschusses Hygiene, Bau und Technik
Empfehlungen der Kommission für Krankenhaushygiene und Infektionsprävention (KRINKO)
GDP
Arzneimittelhandelsverordnung – AM-HandelsV
Leitlinien für die gute Vertriebspraxis von Humanarzneimitteln – GDP
