GxP / Regulatory-Compliance | Qualification as a Part of Pharmaceutical Quality Assurance System
GxP stands for quality management (QM) systems in the areas of pharmaceuticals, chemistry, biotechnology, food (U.S.A.) and medical devices as well as their suppliers and contractual partners.
The pharmaceutical quality assurance system (PQS) represents the entirety of all measures taken to ensure the required quality of medicinal products for their intended use. An effective PQS cannot therefore be a “one-off performance” for, for example, obtaining a manufacturing permit, but must be tracked and continuously improved throughout the entire product life cycle.
An effective PQS should ensure product realization, control and monitoring as well as continuous improvement (GMP Guide Part III, Q10 Note for Guidance on Pharmaceutical Quality System). To implement, maintain and monitor, an effective PQS has the following elements and tools:
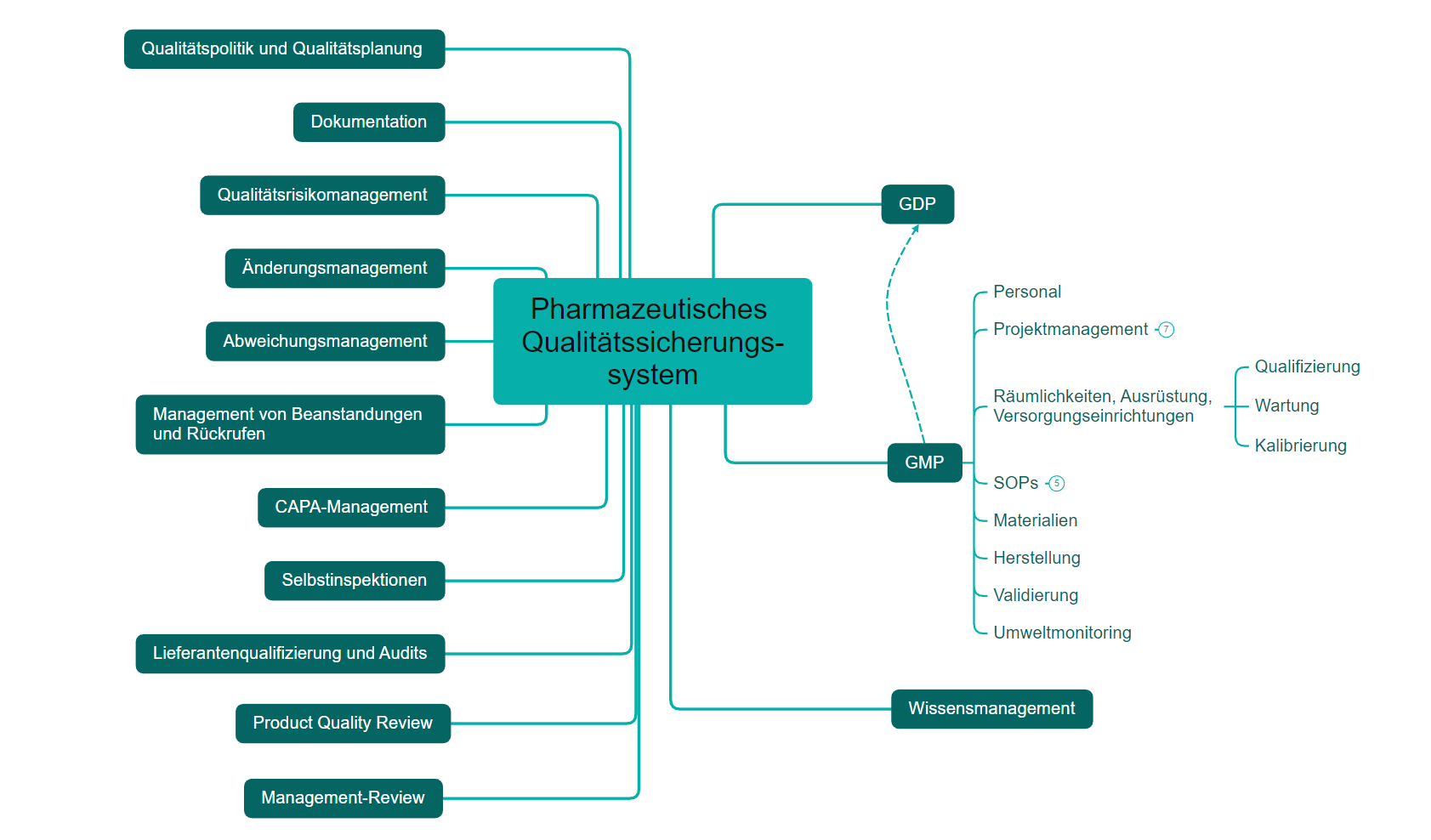
PQS Diagram
We would be happy to support you in developing an effective PQS. This also includes a content review of existing systems and work instructions (SOPs). We convert proven individual work processes into standardized and practice-oriented processes. Our goal is to uncover potential for improvement and to consistently implement a risk-based approach.
Our diverse and practical expertise lies in the areas of traditional pharmacy (GMP, GDP) and medical devices.
We also offer:
• Conduct self-inspections
• Preparation, support and follow-up of official inspections
• Compliance checks to examine manufacturing processes, qualifications and uncover potential for improvement.
Our diverse and practical expertise lies in the area of classic pharmacy (GMP, GDP) and medical devices.
GMP Compliance in Projects through Intelligent Qualification
The manufacture of pharmaceutical products is subject to the highest quality standards, particularly with regard to purity, safety, and traceability.
The qualification of pharmaceutical manufacturing systems, including cleanrooms, supply systems/ultrapure media, production equipment, and computer-aided systems, is carried out specifically in accordance with the requirements of Annex 15 of the EU GMP Guidelines. The goal is to provide documented evidence that the systems used are suitable for meeting the specified requirements reproducibly – in compliance with regulatory requirements and in line with the overarching quality system.
The standard is the Integrated C&Q (Integrated Commissioning & Qualification) approach:
To combine efficiency and compliance as part of the Lean approach, qualification is ideally carried out within the framework of an integrated commissioning and qualification concept (C&Q). In this process, testing activities from the technical commissioning process (commissioning) are used for regulatory purposes (a “leveraging approach”) to avoid repetition and optimize schedules. It is crucial to ensure regulatory traceability.
This approach is not only useful for complex projects with many interfaces and a high degree of automation.
Our approach follows a lean philosophy and is based on risk-based qualification. Through targeted risk analyses (e.g., FMEA), critical qualification activities are identified, non-value-adding steps are eliminated, and test processes are integrated in a lean manner. The clear risk-based structure of the qualification reveals that what does not pose a risk is not subject to any qualification requirements.
A success criterion is also to integrate the acceptance criteria into the risk analysis in line with the defect description. This allows the qualification plans to be created in an integrated manner.
Areas of application:
Facilities – e.g., production areas as clean rooms, storage areas
Utilities – e.g., E.g., HVAC systems, ultrapure media systems (e.g., WFI, AP, pure steam, compressed air, nitrogen, etc.)
Equipment – e.g., mixing vessels, autoclaves, filling systems
Computer systems – e.g., process control systems, SCADA, batch record systems, monitoring systems (including GAMP 5-compliant validation)
The service for your project includes the complete planning, coordination, and documentation of the qualification phases
– from the creation of URS with associated risk analysis, the creation of qualification plans for DQ, IQ, OQ, and PQ, through test execution to the final report – in compliance with applicable standards (EU GMP, ICH Q9/Q10, ISPE, GAMP 5).
You can also contact us for our expertise in:
• Compliance checks to audit manufacturing processes and qualifications
• Detection of improvement potential in your PQS or qualification system
Do you need a manufacturing license?
Do you not yet have a manufacturing license pursuant to Section 13 of the German Medicinal Products Act (AMG) for the manufacture of medicinal products? Is an extension pending?
You will receive expert support in meeting the necessary requirements (see PQS diagram) and in applying for a manufacturing license.
You can also contact us regarding our expertise:
• Conducting self-inspections
• Preparation, support, and follow-up for official inspections
