Reference Project
Medical Device Reprocessing Unit
| Client | m-sas GmbH |
| Industry | Reprocessing of medical devices |
| Project type | New building/ Green Field |
| Role P³ | Project Management, Quality Management, Specialist Planning, Order Management, Construction Coordination |
| Area | approx 1,300 sqm (production area) |
| Duration | 06/2023 – 07/2024 |
| Trades | cleanroom systems, water treatment, ventilation technology, building services |
| Special features | Process equipment already selected, Shell construction status,very short response time |

| Objective | Solution |
| Ensuring cleanroom status |
Use of high-performance solutions HVAC system, state-of-the-art wall system |
|
Optimal room conditioning for operation |
Cooling circuit including a deep well |
| Sustainable and secure energy supply |
PV storage, emergency power generator |
Pharmaceutical gas supply of a university hospital
|
Client |
University Hospital |
|
Industry |
GMP Cleanroom/ Hospital |
|
Project type |
Building in existing buildings |
|
Scope |
Gas distribution networks for N2, LIN, CO2, pharm. CA |
|
Role P3 |
Quality Management |
|
Trade |
Technical building equipment |
|
Status |
ongoing |
|
Achievements |
LIN supply network successfully qualified |

Production of Unit Dose for Closed Loop Medication Management in a University Hospital
|
|
|
|
Client |
University Hospital |
|
Industry |
GMP Cleanroom/ Hospital Pharmacy |
|
Project type |
Building in existing buildings |
|
Size |
86 sqm |
|
Scope |
Manufacture of Unit Dose, ISO 8 |
|
Role P3 |
Concept study, client representation, cleanroom planning/ ventilation / process technology, qualification |
|
Trade |
Functional Performance Specification, Cleanroom Planning, Process Technology Planning |
|
Status |
2024, ongoing |
|
Achievements |
Invitation to tender as client representative (FLB for GÜ), preparation of a market analysis for unit dose machines, workshop on the process flow, developing layout of manufacturing areas |

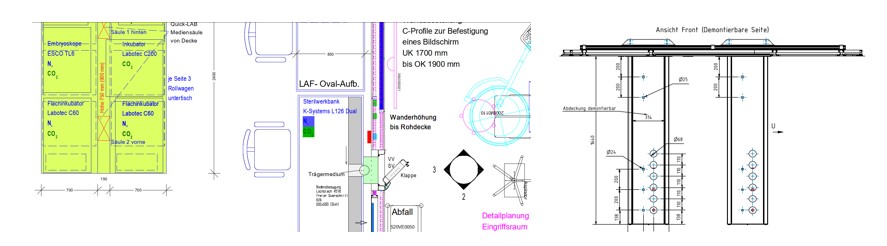
IVF laboratory including pharmaceutical gas supply of a university hospital
|
|
|
|
Client |
University Hospital |
|
Industry |
Hospital |
|
Project type |
Building in existing buildings |
|
Size |
97 sqm (Laboratory, storage, procedure room, airlock, preparation, technical room) |
|
Role P3 |
Project management, quality management, planning phases 2-8, order management, construction coordination/site supervision phase 8, Qualification (URS, RA, DQ, IQ, OQ) |
|
Trade |
Cleanroom systems, air handling technology, furniture, air technology |
|
Status |
2023/2024 |
|
Achievements |
Integration of a laboratory with GMP requirements into hospital environment, optimization of the functionality and work processes of the IVF laboratory |